• There is a layer of ozone in the stratosphere. The ozone layer prevents high energy UV
light from reaching the troposphere.
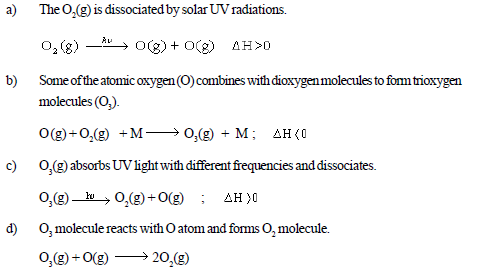
• Some reactions involving O2(g) and O3(g) are;
• Ozone is destroyed by reactions with and other free radicals. These radicals
act as catalysts and destroy thousands of O3 molecules. The catalysed destruction of
ozone in the stratosphere is known as ozone layer depletion.
• Chlorine radicals from the chlorofluorocarbon have been recognized as a major contribution
to ozone layer depletion . This chloroflurocarbons are stable in the atmosphere but produce
radicals in stratosphere with UV radiation.
• There is a strong connection between UV radiation and the cataract formation as well as
incidence of both non-fatal and fatal skin cancer in humans. The ozone layer protects us.




Comments
Post a Comment